B.Pharmacy 7th Semester Notes
UV Visible Spectroscopy
Instrument method of analysis

Download PDF
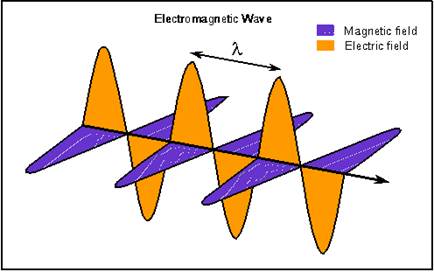
Electromagnetics Wave
- E = hν = h c / λ
- Wavelength (λ) = 1 / ū = c / ν
- Wave-number (ū) = 1 / λ = ν / c
- Frequency (ν) = c / λ = c ū
- Velocity (c) = νλ = ν / ū
- 1 Ǻ = 10-1 nm = 10-8 cm
Electromagnetic spectrum

Electronic Excitation by UV/Vis Spectroscopy
Introduction
A. UV radiation and Electronic Excitations The difference in energy between molecular bonding, non-bonding and anti-bonding orbitals ranges from 125-650 kJ/mole. This energy corresponds to EM radiation in the ultraviolet (UV) region, 100-350 nm, and visible (VIS) regions 350-700 nm of the spectrum
B. The Spectroscopic Process
- In UV spectroscopy, the sample is irradiated with the broad spectrum of the UV radiation.
- If a particular electronic transition matches the energy of a certain band of UV, it will be absorbed.
- The remaining UV light passes through the sample and is observed.
- From this residual radiation a spectrum is obtained with “gaps” at these discrete energies – this is called an absorption spectrum.

C. Observed electronic transitions
- The lowest energy transition (and most often obs. by UV) is typically that of an electron in the Highest Occupied Molecular Orbital (HOMO) to the Lowest Unoccupied Molecular Orbital (LUMO)
- For any bond (pair of electrons) in a molecule, the molecular orbitals are a mixture of the two contributing atomic orbitals; for every bonding orbital “created” from this mixing (σ, π), there is a corresponding anti-bonding orbital of symmetrically higher energy (σ, π)
- The lowest energy occupied orbitals are typically the σ; likewise, the corresponding anti-bonding σ* orbital is of the highest energy
- p-orbitals are of somewhat higher energy, and their complementary anti-bonding orbital somewhat lower in energy than σ.
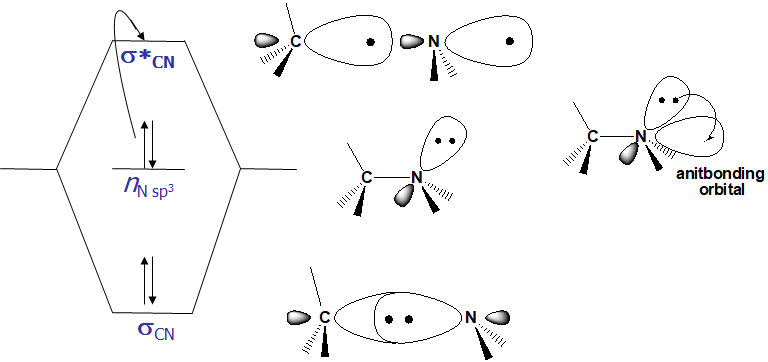
- Unshared pairs lie at the energy of the original atomic orbital, most often this energy is higher than π or σ (since no bond is formed, there is no benefit in energy)
- Here is a graphical representation

- From the molecular orbital diagram, there are several possible electronic transitions that can occur, each of a different relative energy:

- Although the UV spectrum extends below 100 nm (high energy), oxygen in the atmosphere is not transparent below 200 nm.
- Special equipment to study vacuum or far UV is required.
- Routine organic UV spectra are typically collected from 200-700 nm.
- This limits the transitions that can be observed:

D. Selection Rules
Not all transitions that are possible are observed.For an electron to transition, certain quantum mechanical constraints apply – these are called “selection rules”.For example, an electron cannot change its spin quantum number during a transition – these are “forbidden”.Other examples include:the number of electrons that can be excited at one timesymmetry properties of the moleculesymmetry of the electronic statesTo further complicate matters, “forbidden” transitions are sometimes observed (albeit at low intensity) due to other factors
E. Band Structure
Unlike IR (or later NMR), where there may be upwards of 5 or more resolvable peaks from which to elucidate structural information, UV tends to give wide, overlapping bandsIt would seem that since the electronic energy levels of a pure sample of molecules would be quantized, fine, discrete bands would be observed – for atomic spectra, this is the caseIn molecules, when a bulk sample of molecules is observed, not all bonds (read – pairs of electrons) are in the same vibrational or rotational energy states This effect will impact the wavelength at which a transition is observed – very similar to the effect of H-bonding on the O-H vibrational energy levels in neat samplesWhen these energy levels are superimposed, the effect can be readily explained – any transition has the possibility of being observed.

Chromophores
A. Definition
- Remember the electrons present in organic molecules are involved in covalent bonds or lone pairs of electrons on atoms such as O or N.
- Since similar functional groups will have electrons capable of discrete classes of transitions, the characteristic energy of these energies is more representative of the functional group than the electrons themselves.
- A functional group capable of having characteristic electronic transitions is called a chromophore (color loving).
- Structural or electronic changes in the chromophore can be quantified and used to predict shifts in the observed electronic transitions
- B. Organic Chromophores
- Alkanes – only posses σ-bonds and no lone pairs of electrons, so only the high energy σ → σ transition is observed in the far UV. This transition is destructive to the molecule, causing cleavage of the σ-bond.

- Alcohols, ethers, amines and sulfur compounds – in the cases of simple, aliphatic examples of these compounds the n → σ* is the most often observed transition; like the alkane σ → σ* it is most often at shorter λ than 200 nm. Note how this transition occurs from the HOMO to the LUMO.