- Define surfactants.
- Define CMC (critical miscelleconcentration).
- Explain the mechanism of formation of micelles with diagram.
- Classify surfactants with suitable examples.
- Give medical applications of surfactants.
- Give pharmaceutical applications of surfactants.
- Explain how cationic amphiphiles posses antibacterial activity against gram negative organisms.
- Explain how amphiphiles above CMC decrease effectiveness of hexylresorcinol towards pinworms.
Solutions:
Contents
1. Define surfactants.
2. Define CMC (critical miscelle concentration).
Critical micelle concentration or cmc, defined as the concentration range of a surfactant at which micelles start forming.
Cmc is a concentration range and has unit of concentration such as w/w, w/v percent, moles/liter, moles/1000 g of solvent etc.
3. Explain the mechanism of formation of micelles with diagram.
The concentration ar which micelle formation occurs is termed critical micelle concentration (CMC). In the micelle, the surfactant hydrophobic groups are directed towards the interior of the aggregate and the polar head groups are directed towards the solvent thus micelles help in solubilization of the dispersed phase.
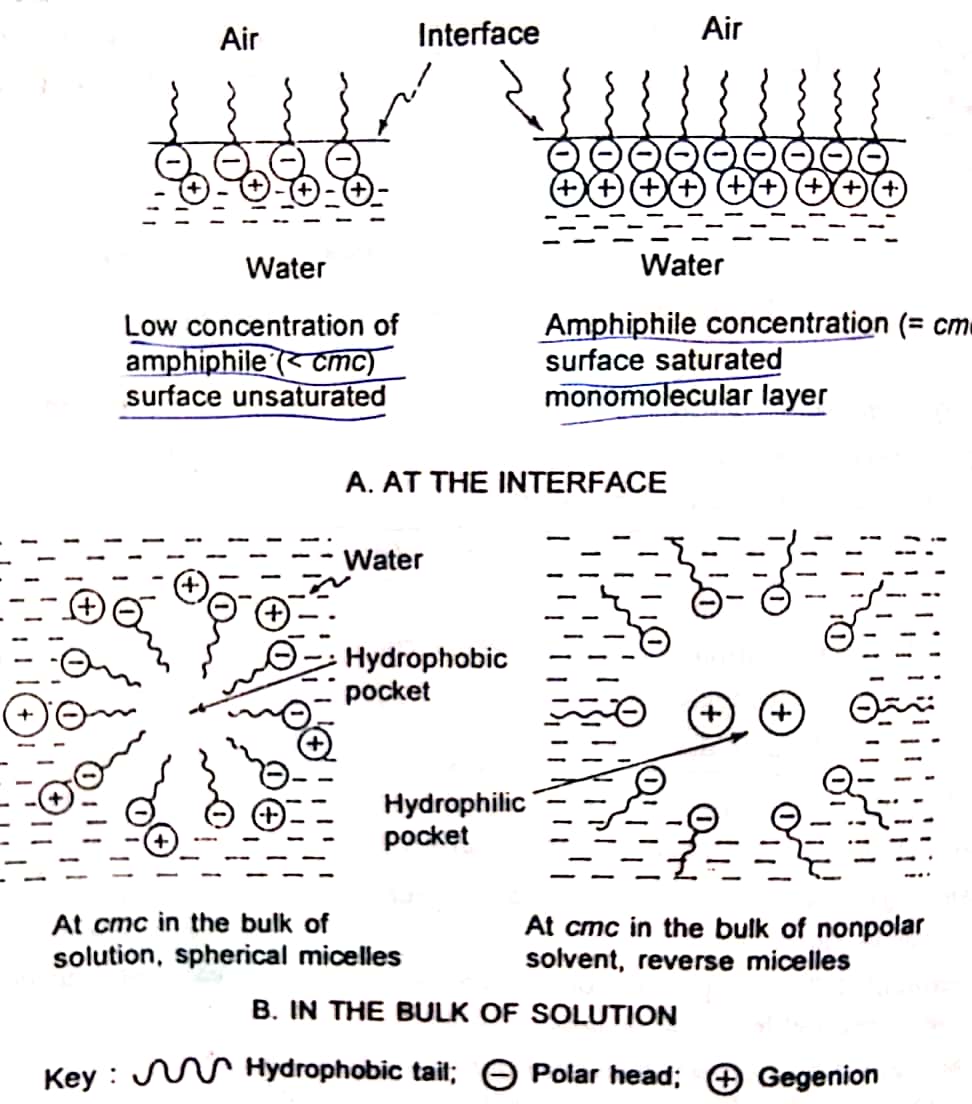 |
| The behavior of surfactant molecules in aqueous and nonpolar media. sodium lauryl sulphate is used for representation. |
 |
| At the Interface |
 |
| In the bulk of solution |
 |
| In the bulk of the solution |
4. Classify surfactants with suitable examples.
Classification of Surfactants.
- Anionic Surfactants
- Alkali soaps (sodium and potassium stearate):
- Sodium, potassium and ammonium salts of long-chain fatty acids (stearic and oleic acid)
- Unstable below pH 10 and are incomplete with acids and polyvalent inorganic and long-chain organic cations.
- Metallic soaps (calcium stearate)
- Salts of divalent and trivalent metals (calcium, magnesium, zinc and aluminium) with long-chain fatty acids
- Amine soaps
- Formed by reaction between amines (ethanolamine, diethanolamine and triethanolamine) and fatty acids (oleic acid)
- Alkyl sulphates and phosphate (sodium lauryl sulphate)
- Esters formed by reaction of fatty alcohols with sulphuric acids and phosphoric acid
- Alkyl sulphonates (sodium dioctyl sulphosuccinate also known as aerosol AT)
- Effective wetting agent
- Cationic Surfactants
- In aqueous solutions, cationic surfactants dissociates to form positively charged cations, which give them emulsifying properties ammonium compounds such as cetyltrimethylammonium bromide (cetrimide), benzethonium chloride and benzalkonium chloride are examples of important cationic surfactants.
- Ampholytic Surfactants
- Ampholytic surfactants posses both cationic and anionic groups in the same molecule and their ionic characteristics depends on the pH of the system. Below a certain pH value they behave as cations, above a certain pH value they behave as anionic, and at intermediate pH, they behave as zwitterions. Examples of ampholytic surfactants include lecithin and N-dodecyl alanine.
- Lecithin is used as emulsifier for parenteral applications.
- Nonionic Surfactants
- Unlike anionic and cationic surfactants, nonionic surfactants are useful for oral and parenteral formulations because of their low irritation and toxicity. Based on their neutral nature, they are much less sensitive to changes in the pH of the medium and the presence of electrolytes. These are available in various hydrophilic-lipophile balances (HLBs), which stabilize oil-in-water (O/W) or water-in-oil (W/O) emulsions
- Sorbitan ester(Spans)
- Polysorbates (Tweens)
- Polymeric Surfactants
- The most commonly used polymeric surfacts used in pharmacy are the A-B-A block copolymers, with A being the hydrophilic chain [poly(ethylene oxide), PEO] and B being the hydrophobic chain [poly(propylene oxide), PPO]. The general structure is PEP-PPO-PEO and is commercially available with different with different propertions of PEO and PPO (Pluronics, Synperonic and Poloxamers). Another impotant class of polymeric surfactant that are used for demulsification is those based on alkoxylated alkylphenol formaldehyde condensates. Silicone surfactants with a poly(dimethyl siloxane) backbone can cause enhanced wetting and spreading of their aqueous solution.
- Used to prepare highly stable concerned suspensions.
5. Give medical applications of surfactants.
Medical applications of Surfactants:
- As Antimicrobials: Cationic surfactants such as cetrimide and benzalkonium chloride have useful antibacterial properties. They are used as disinfectants for instruments and as an antiseptic for the skin. These surfactants adsorb over the surface of the bacterial cell (Gram-negative bacteria) owing to their positive charge. THis changes the cell membrane permeability, resulting in loss of essential substances from the cell, in turn resulting in its death.
- As expectorants: In acute and chronic infections of the upper respiratory tract (e.g. bronchitis, asthma and TB), the viscosity of bronchial mucuc increases. The mucus dries out, which cause difficulty in breathing. Inhalation of sprays or mists (aerosols) containing surfactants, such as calfactants, loosen the mucus and results in its easy removal, thereby providing relief.
- As cleansing agents: Since surfactants have detergents properties, these are also used as cleansing agents. However, their repeated use should be avoided since this may cause irritation of the skin. Examples of surfactants used as cleaning agents include ammonium lauryl sulphate.
6. Give pharmaceutical applications of surfactants.
Pharmaceutical applications of Surfactants:
As solubilizing agents: Surfactant have been extensively used as solubilizing agent for number of poorly soluble drugs such as oil-soluble vitamins, volatile oils, hormones, phenobarbitone and sulphonamides. Oil-soluble vitamins such as Vitamin A are unpleasant to take in the form of fish liver oil but are easily palatable when administered in the form oil in water emulsions or as solubilized system in water. Such solubilized systems are more resistant to oxidation than either oily solutions or emulsions. Surfactants have also been used to solubilize many disinfectant compounds such as cresol and chloroxylenol. Lysol which is a solution of phenol and alkali soap in water, is a very good disinfectant. The disinfectant property of the compound is increased by the use of surfactants since they also alter the permeability of the cell membrane of microorganisms.
As wetting agents: Their hydrophobic nature makes them aggregate and agglomerate when added to water. Dispersions containing such hydrophobic powders are often difficult to prepare since the powders for large floccules or float on the surface, thereby hindering the preparation of a uniform suspension. This can be solved by the use of surfactants which get adsorbed at the solid/liquid interface and increase the affinity of the hydrophobic powder for water while reducing the attractive forces between particles of the solid. Aerosol OT is a very good example of wetting agent.
As flocculating agents: Use of surfactants coupled with precipitation results in the desirable action of controlled flocculation in suspension. For example, sulfamerazine, a hydrophobic powder, can be dispersed by means of aerosol OT in association with aluminium ions. Although these flocculated particles settle on standing, they do not form a hard cake and easily disperse in the vehicle on shaking.
As additives in semisolid preparations: Surfactants are often added to creams and ointments to alter the release characteristics of the incorporated drug. The release rate may be accelerated because of the absorption of water from the surrounding environment. Further, the capacity of different ointment bases to take up aqueous liquids can also be improved by the addition of surfactants.
7. Explain how cationic amphiphiles posses antibacterial activity against gram negative organisms.
Antibacterial activity of Surfactant: Ionic surfactants adsorb on the cell surface by electrostatic interaction. As a result, the cell surface looses its integrity and the essential materials are lost through the leaks. Thus antibacterial action is observed. Both gram-negative and gram-positive organisms are susceptible to the action of cationic quaternary com pounds, whereas gram-positive organisms are attacked more easily by anionic agents than gram-negative organisms. Non-ionic agents are least effective as antibacterial agents. In fact, they often help in the metabolism of the organisms and facilitate their growth.
8. Explain how amphiphiles above CMC decrease effectiveness of hexylresorcinol towards pinworms.
Surfactants at low concentrations enhance the penetration of hexylresorcinol into the pinworm, Ascaris.They reduce the interfacial tension between the liquid phase and the cell wall of the organism. As a result, the drug is absorbed rapidly and bring about the cell death. When the concentration of surfactant exceeds cmc, the rate of absorption of the hexylresorcinol decreases. In this situation, the drug is partitioned between the aqueous phase and micelles. Thus, less amount of drug is available for absorption.