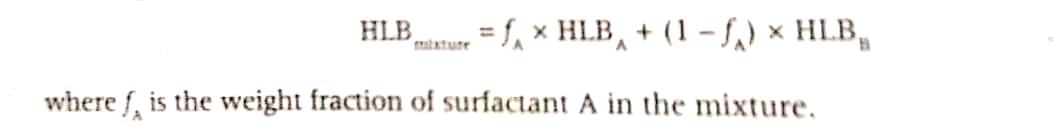Some Important Questions from Surface and Interfacial Phenomenon as per B Pharmacy PCI Pattern are:
- Who introduced the HLB concept and define HLB scale?
- Draw HLB scale with values of various surfactants along with examples.
- Enumerate various formulas for calculation HLB values.
- Explain RHLB (Required HLB).
- What are the various steps involved in detergency. Explain it with flow chart diagram.
- Write a detailed note on HLB and give its two limitations.
Questions with Solutions:
Contents
1. Who introduced the HLB concept and define HLB scale?
Since several applications are reported, one has to be cautious in the selection of a surfactant. Keeping in view of innumerable number of surfactants are available, selecting a right kind or type of surfactant for intended purpose is a difficult task. Selection by trial and error is costly and impracticable. One empirical selection system is HLB as proposed by Griffin in 1947.
HLB is an arbitrary scale that indicates the extent of hydrophilic lipophilic balance (HLB). Surfactants such as Spans (sorbitan ester) are Lipophilic and have low HLB values (1.8 to 8.6). On the other hand, Tweens (polyoxyethylene derivatives of Spans) are hydrophilic and have high HLB values (9.6 to 16.7). In general, the higher the HLB of an agent, the more the hydrophilicity. A HLB value of one (1) indicates that the surfactant is soluble in oil. a HLB value of 20 implies that it is soluble in water. The HLB scale is used to identify the optimum efficiency of a variety of surfactants.
2. Draw HLB scale with values of various surfactants along with examples.
Here this diagram shows the HLB scale.
According to this diagram, The higher the HLB number, the more hydrophilic is the surfactant. The Lower the HLB number, the more lipophilic is the surfactant. Exceptions to HLB scale such as sodium lauryl sulphate with an HLB value of 40.
3. Enumerate various formulas for calculation HLB values.
The Calculations of HLB value are as follows:
- HLB Values of surfactants based on polyhydric alcohol fatty acid esters such as glyceryl monostearate, sorbitan monooleate and polyoxyethylene sorbitan monooleate may be estimated by the following equation:
- HLB = 20*(1-Saponification number of ester)/Acid Number of the fatty acid.
- For material such as beeswax and lanolin derivatives with which it is not possible to obtain good saponification number, the HLB value is estimated by the following.
- HLB = (Weight percent of oxyethylene chain + Wt. percent of polyhydric alcohol group in the material.) / 5
- For material whose hydrophilic region is polyoxyethlene, the HLB value is calculated by the following:
- HLB = Weight percent of oxyethylene chain / 5
- In another method for calculating the HLB values, the component groups of the surfactant molecules are assigned group numbers and these are then added to give the HLB value of the surfactant molecules.
- HLB = Σ (hydrophilic group number) – Σ (lipophilic group numbers) + 7
4. Explain RHLB (Required HLB).
This means that a surfactant, or blend of surfactants having an HLB of 10 will generally make a more stable & fluid O/W emulsion with mineral oil than surfactants of any other HLB.
Generally, a single emulsifier cannot yield the desired type of emulsion. More often, sable emulsions can be prepared by utilizing a combination of a hydrophilic and a lipophilic surfactant. Such combinations produce mixed interfacial phases of high surface coverage as well as of sufficient viscosity to prevent creaming.
HLB values of combinations of surfactants A (HLBA, and B (HLBB) are calculated by the following equattions:
For example a W/O emulsion of lanolin requires an HLB of about 8.0. Thus a 68.32 mixture of span (HLB 4.7) and Tween 20 (HLB 15.0) could be used to yield an emulsifier with an average HLB value of about 8.0.
5. What are the various steps involved in detergency. Explain it with flow chart diagram.
Surfactants in aqueous solutions are used to remove the dirt from substrates such as glass, fabric, skin etc. Effective detergents are required for the cleaning of production equipment, containers for packing and also in order to maintain hygiene in the industry. Detergency is a complex process and a number of steps are simultaneously involved. These are:
- initial wetting of the dirt from the surface
- solubilising of the dirt
- removing the insoluble dirt as deflocculation particles
- suspending the particles in the detergent solution
- removing the oil soluble materials and convert into emulsion
- converting the dirt into foam so as wash easily
 |
| FIgure: The phenomenon of detergency showing the number of processes |
The HLB requirement for the detergent is about 13 to 16. Some examples of detergents of ionic type are:
Cationic type :
Zephiran (benzyl dimethyl cetyl ammonium chloride) &
Cetrimide (cetyltrimethyl ammonium chloride)
Anionic Type:
Soaps, Sodium lauryl sulphate.
6. Write a detailed note on HLB and give its two limitations.
Limitations of HLB are:
- HLB system provides information regarding the nature of the surfactant used, but the concentration of such surfactant is equally important. The HLB system neglects the concentration of surfactant required for the optimum stability of emulsions.
- Though HLB of a surfactant indicates the solubility properties, it is an over-simplification. It cannot be always realistic. Because, in general, solubility depends on the nature of solvent, temperature and presence or absence of additives.
- HLB numbers of surfactant blends (nonionic) are surprisingly additive according to the proportions of each surfactant, though the origin of HLB system is empirical.