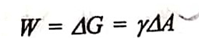Important Questions:
- Define Adsorption. Difference between physical and chemical adsorption.
- Explain how solid surfaces offer a site for adsorption of gases & dissoluted solute through concept of surface tension.
- Give thermodynamic considerations for adsorption process.
- Define adsorption isotherm. Explain Freundlich isotherm with equation & plot.
- What are the factors which affect adsorption of solute molecules from its solution?
- Comment : Adsorption is surface phenomenon & absorption is internal phenomenon.
- Explain positive and negative adsorption with example.
- Define desorption.
1. Define Adsorption. Difference between physical and chemical adsorption.
Adsorption is the process in which materials of one phase (adsorbate) accumulate or concentrate at the interfacial surface of the other phase (adsorbent). It is a spontaneous phenomenon driven by a reduction of the surface free energy. Adsorption occurs at the interfaces of two phases such as liquid/liquid, gas/liquid, liquid/solid or gas/solid.
Two different types of adsorption processes exist: physical adsorption (physisorption) and chemical adsorption (chemisorption).
In physical adsorption, adsorbates adsorb on the surface of a solid by van der Waals forces, which are relatively weak and nonspecific forces. The adsorbate is not fixed to the surface of the solid and can move freely within the interfacial surface. Physical adsorption is fast, reversible and results in multilayer adsorption.
In chemical adsorption, the substance is held on the surface of a solid by specific covalent forces between the adsorbate and the adsorbent. The chemically adsorbed materials are not free to move on the surface. Chemical adsorption is slow, not readily reversible and results in monolayer adsorption.
|
Parameter
|
Physical adsorption
|
Chemical adsorption
|
|
Force
|
Weak van der Waals forces
|
Strong forces
|
|
Specificity
|
Nonspecific
|
Specific
|
|
Reversibility
|
Reversible
|
Often irreversible
|
|
Effect of temperature
|
Exothermic, adsorption
|
Surface reaction proceeds
|
|
Adsorbed layers
|
decreases as temperature increases Multilayer formation
|
above certain temperature
Monolayer formation
|
Adsorption is a dynamic phenomenon that is opposed by desorption, i.e. the transfer of a surfactant to a bulk phase. The adsorption and desorption steps are often very rapid; Consequently, adsorption—desorption equilibrium is reached after some time, which depends on the surfactant concentration in the bulk phase. Since the surfactant has a lower free energy when it is adsorbed at the interface than in the solvent bulk phase, the equilibrium is displaced towards the adsorbed state. In fact, the interface is rapidly covered by a monolayer of surfactant and everything happens as if the interface is coated with a thin layer of a new material.
2. Explain how solid surfaces offer a site for adsorption of gases & dissolved solute through concept of surface tension.
According to the concept of surface tension, molecules at the surface are in an unbalanced state. The same is true regarding the surface of a solid. It means molecules or ions that are present at the surface of a solid do not have all their forces satisfied by attractions of other particles (or molecules). Therefore, solid and liquid surfaces tend to attract gases or dissolved substances and retain them on their surfaces, when they come in contact.
3. Give thermodynamic considerations for adsorption process.
The thermodynamic considerations for the adsorption process is as follows. Adsorption is entirely a surface phenomena. The greater the surface area, the greater is the adsorption. when a substance is subdivided into a finer state, the surface free energy increases. It is related to surface tension as per equation represented as follow:
where W = work done to obtain division of particles,
ΔA = increase in the surface area,
ΔG = increase in the surface free energy.
The enhanced surface area increases the surface free energy. Hence, the system (powder or adsorbent) becomes thermodynamically unstable. Now, the system tends to minimize surface energy and regain stability. Under the experimental conditions for adsorption, AA cannot be decreased. Therefore, the reduction in the surface free energy means a reduction in the surface tension.
A reduction in surface free energy takes place by the movement of solute ions, atoms or molecules on to the surface of particles and form a film around it. The reduction. in surface energy accounts for the phenomena of adsorption. In this process, the system assumes thermodynamic stability.
Adsorption should not be confused with absorption. The term absorption is applied to the phenomenon, when molecules enter the body of the liquid or solid as in the case of absorption of gas by a liquid. For example, dissolving (adsorption) carbon dioxide gas in water as in the case of carbonated beverages. The term adsorption is applied, when molecules act on the surface of the body. Sometimes, it is difficult to differentiate absorption and adsorption. For example, interaction Of substances with highly porous solids. In such cases of difficulty, a term ‘sorption’ is used.
4. Define adsorption isotherm. Explain Freundlich isotherm with equation & plot.
In the study of adsorption, the amount of gas adsorbed per unit area or unit mass of solid is measured at different pressures of the gas. The study is usually conducted at constant temperature and graphs are plotted. These plots are called adsorption isotherms.
Freundlich isotherm : The relationship between pressure of the gas and amount adsorbed at constant temperature has been expressed by many equations. For Freundlich isotherm, the equation is:
where x = weight of gas adsorbed per unit weight of adsorbent, m
P = equilibrium pressure
k and n = constants
The above equations gives curvilinear graph when (x/m) is plotted against pressure, P.
 |
| Adsorption isotherm for a gas on za solid. Freundlich adsorption isotherms. Curvilinear plot drawn as per equation |
The constants, ‘k’ and ‘n’, are evaluated from the results of the experiment and depend on the temperature and the nature of adsorbent and adsorbate. The constants can be easily obtained by converting the equation into logarithmic form
Thus, plotting log (x/m) versus log P gives a straight line with the slope (l/n) and the intercept on the y axis is k.
 |
| Adsorption isotherm for a gas on za solid. Freundlich adsorption isotherms log-log plot drawn according to equation. Amount of ‘x’ of gas adsorbed per unit weight (m) adsorbent and ‘P’ is the equilibrium pressure. |
Gases such as carbon dioxide, sulfur dioxide, hydrochloric acid and ammonia which are liquefiable get more readily adsorbed than other permeant gases such as oxygen, hydrogen and nitrogen. In general, the higher the critical temperature of the gas, more is the adsorption of the gases.
5. What are the factors which affect adsorption of solute molecules from its solution?
Factors Affecting Adsorption are:
The adsorption of solute molecules from its solution may be influenced by the following factors:
- Nature of adsorbent: The physicochemical nature of the adsorbent can have decisive impact on the rate and capacity for adsorption. Every solid material can be used as an adsorbent, but activated carbon and clays such as kaolin and bentonite have been used as particular adsorbents in pharmaceutical applications.
- Nature of adsorbate: The solubility of the adsorbate in the solvent from which adsorption takes place has an inverse relationship with the extent of adsorption (Lundelius’ rule). The forces between the adsorbate and solvent need to be broken for adsorption to occur. Thus, higher the solubility of the adsorbate in a solvent, the greater the forces and the smaller the extent of adsorption.
- Adsorbent—solute interaction: Adsorption of a solute from a dilute solution involves the breaking of bonds between the solute and the solvent molecules as well as the formation of bonds betweenthe solute and adsorbent molecules. As an example, the higher molecular weight solutes are usually more readily adsorbed than low molecular weight solutes. This is due to van der Waals forces of attraction, which increases with the size of molecules.
- Adsorbate concentration: The amount of adsorption increases with the increase in the concentration of solute at equilibrium until it reaches a limiting value. However, the relative amount of solute removed from the solution is greater in dilute solutions.
- Surface area of adsorbent: Adsorption is a surface phenomenon and the amount of solute adsorbed depends on the surface area available. Thus, reducing the particle Size of the adsorbent will increase the adsorption.
- Temperature: Physical adsorption is an exothermic process and thus a decrease in temperature will increase the extent of adsorption.
- Removal of adsorbed impurities: Removal of adsorbed impurities such as gases or moisture from the surface of solid adsorbent activates the active adsorption sites and increases the efficiency of adsorbents. This can be achieved by heating the adsorbent at high temperature (at 110°C for 1 hour).
- pH of the medium: pH of a solution influences the extent of adsorption since pH affects both the degree of ionization and the solubility of the adsorbate drug molecule. More ionized (i.e. polar) and soluble adsorbates adsorb much less than their unionized forms (i.e. lipophilic). Amphoteric adsorbates such as proteins are usually best adsorbed at the isoelectric point where the net charge of the adsorbate becomes zero, and at the lowest solubility.
6. Comment : Adsorption is surface phenomenon & absorption is internal phenomenon.
Absorption involves the penetration of liquid or gas into the capillary spaces of the absorbing medium. Adsorption involves the settling (or deposition) of liquid or gas on the surface of the adsorbent. As per definition, absorption is an internal phenomenon, while adsorption is a surface or interfacial phenomenon.
7. Explain positive and negative adsorption with example.
Certain molecules or ions when added to a liquid, they may modify the interface in different ways. When the added molecules move on their own accord to the interface, this process is called positive
adsorption or simply adsorption. Examples are surface active agents such as sodium lauryl sulfate, Tweens and triethanolamine. They partition in favour of interface and reduce the interfacial (or surface) tension.
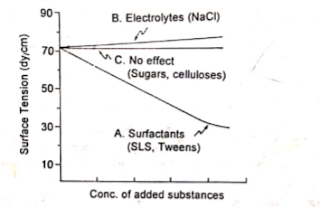 |
| Influence of added substance on the surface tension of water |
Certain molecules prefer to remain in the bulk of solution. Such process is referred to as negative adsorption. Though they remain in the bulk, still they affect the interfacial properties. For example, when inorganic electrolytes (sodium chloride) are added to water, they marginIlly enhance be surface tension. The electrolytes remain in in the bulk and pull the solvent molecules on the surface through electrostatic interactions.
Some substances do not affect the interfacial tension when they are added to water. Examples are: sugars, carbohydrates and cellulose derivatives. Any marginal increase in the interfacial tension may enhanced viscosity of sugar solutions.
Adsorption should not be confused with absorption. Adsorption is solely a surface effect, whereas in absorption, the liquid or gas being into the capillary spaces of the absorbing medium.
8. Define desorption.
It is difficult to clearly identify the type of adsorption, but a combination of both types of adsorptions may be observed. In such cases, a term sorption is used. The phenomenon oppsite to adsorption is termed as desorption. In this process, the adsorbed molecules or ions are removed from the solid surface.